Water Quality Standards for Agriculture: An Underestimated Factor in The Spray Tank

Water quality for agriculture is a topic frequently discussed at farmer gatherings, on the farm or at training events. There is a lack of understanding when it comes to the effect of water quality on the chemicals that are utilised. Typically, only pH is considered and direct connections can then supposedly be derived between pH and salinity indices. This can be totally misleading. Very expensive decisions that can impact crop yields are made about spray mixtures without giving sufficient attention to the specific product requirements.
Additionally, the contributing effects of certain cations is also frequently overlooked. This article aims to shed more light on the interpretation and application of water quality data for pest control purposes.
This article does not attempt to articulate every possible scenario, and only discusses basic principles. Specific technical enquiries must be directed to the manufacturer if the product label and directions for use does not address them.
What is important?
- Units of measurement.
- Electrical Conductivity (EC) and total dissolved solids (TDS).
- pH.
- Hardness, cations, bicarbonate and carbonate.
- Turbidity
- Water temperature.
Discussion of water quality parameters
The units will be discussed under the relevant headings.
Electrical conductivity and total dissolved solids
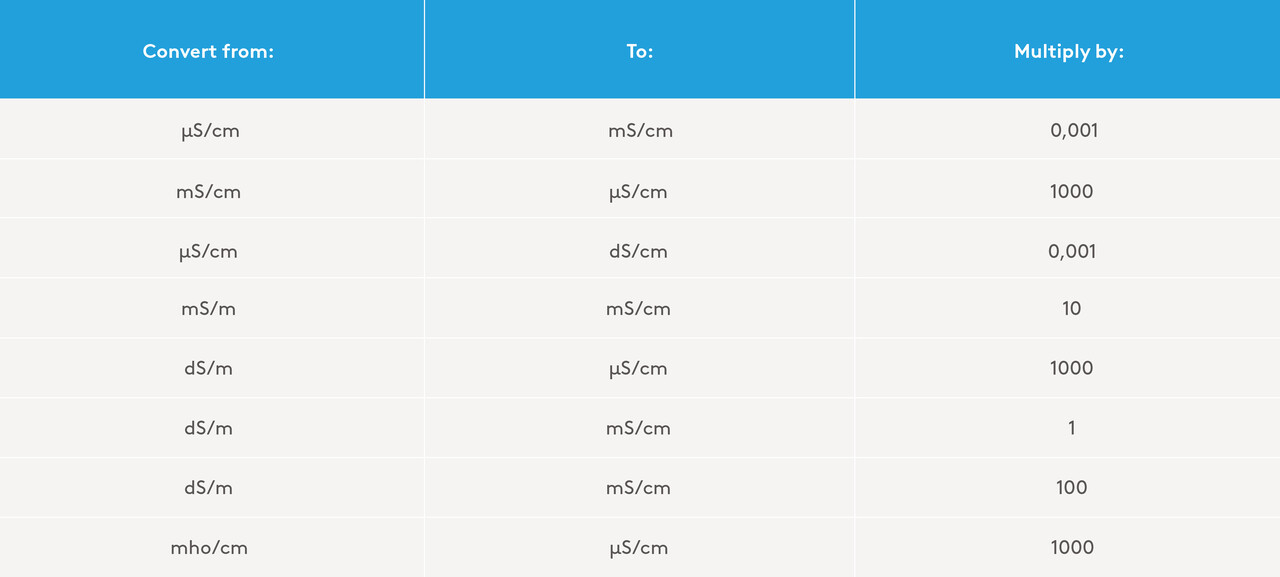
“Electrical conductivity (EC)” measures the ability of the water resources to conduct electricity. This is measured by inducing a constant potential difference (in volts) between two fixed metallic points 1cm apart. The strength of the current is thus an indication of the conductivity of the water. Pure water has zero conductivity because it does not contain any dissolved salts. The unit can then be described as milliSiemens per linear centimetre — mS/cm. Many laboratories report this unit as µS/cm, occasionally also mS/m. The old unit of measurement may also be noted frequently i.e., mho/cm (millimho per centimetre).
Table 1: Conversion between different units of measurement for conductivity
This is the opposite of the term ‘ohm’ which measures resistance. The lower the conductivity, the higher the resistance. To make things even more confusing, some laboratories use decimeterSiemens per meter (dS/m). The table below lists the most common measurement units and their conversions:
Total dissolved solids (TDS) is the measure of substances that have completely dissolved in the water. It is expressed as parts per million (ppm) or mg/L in the case of liquids where ppm = mg/L.
An approximate value of the EC or TDS can be calculated using the following formula:
TDS (mg/L) = k x EC (μS/cm) (1)1
The value of the constant (k) varies depending on the quality of the water. For water with an EC between 100mS/m and 500 mS/m, k can be assigned a value of 6.5.
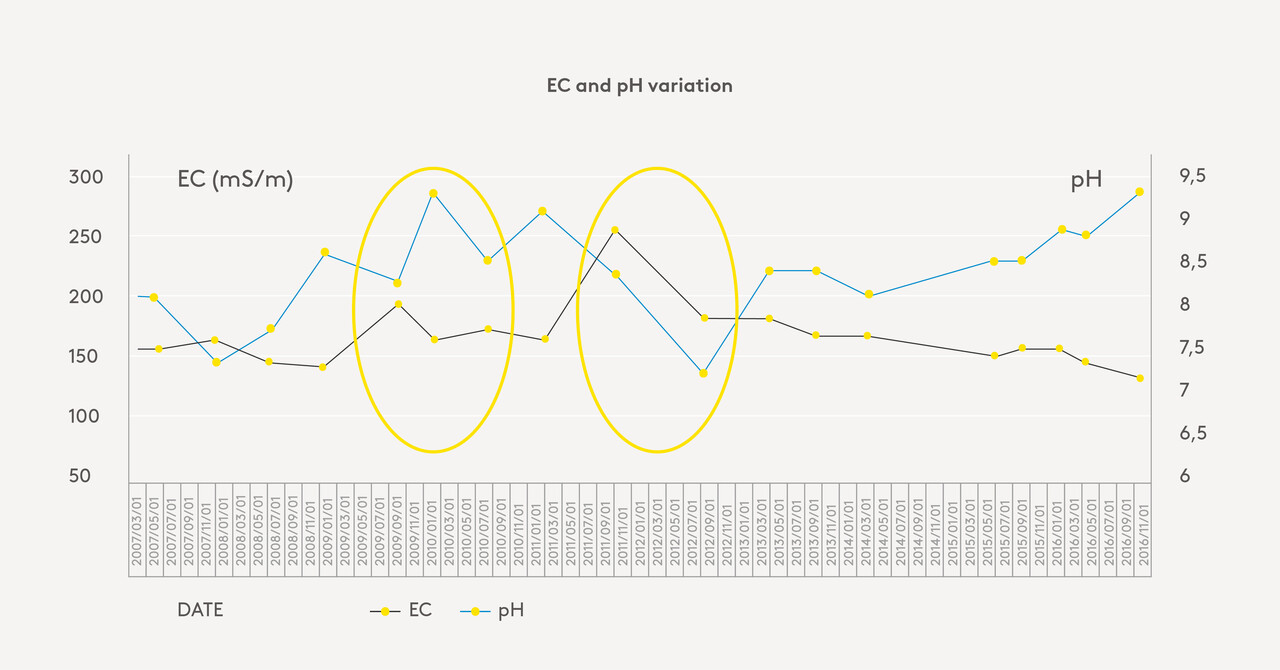
For pure water, you will use a constant (k) of 5. Chloride (Cl-), sodium (Na+) and magnesium (Mg2+) exert the greatest influence on the k value. From the graph [mention of placement eg. above/below] it can be clearly seen that there is a weak relationship between EC and pH. High pH does not necessarily indicate high EC values. The salts responsible for EC and pH can differ significantly.
The influence of EC on chemicals particularly affects herbicides such as 2,4 DB, fluroxypyr and triazines. This is especially valid where the sodium and chlorine each exceed 500 mg/L, or the sum of the two is 1,000 mg or greater, or the EC is above 500 mS/m. TDS values above 1,000 mg/L inhibit mesotrione action by 18% on Palmer-compost mixtures.
Some products may not be salt-sensitive, however, the formulation of the product may well be salt sensitive which result in precipitation (precipitates). Certain Diuron, 2,4-D-, sulfonylureas and MCPA mixtures may be susceptible to this.
Figure 1: Graph of actual readings EC and pH (own data):
Insecticides and fungicides are usually far less sensitive to high EC values. Additives such as oils and certain wetting agents can, however, be affected where the EC becomes high.
Solutions for waters with a high EC can be challenging because there are salts in the solution. Selecting a water source of a higher quality (even if further from the land) or combining with better water sources can alleviate the water quality problems.
Acidity of the spray water expressed as pH
The pH is measured by definition as the concentration of the hydrogen ion on a logarithmic (log) scale. A log scale was selected because the values are very small and multiples of 10 multiplied by 10 become easier. Let us look at an example:
Water is a balance of the hydrogen ion as H3O+ and the hydroxyl ion (OH-) which binds and unbinds millions of times per second to form water, H2O.
H3O+ + OH- = 2H2O (2)
pH = - log [H3O+] (3)
pH = - log [1 x 10-7]
pH = 7
The concentration is therefore 0,0000001 mg/L of the hydrogen ion. Where the composition of the salts dissolved in the water favour reactions where more hydrogen ions are formed, the pH of the water drops.
If we suppose that an acid is added to the water and the concentration of H3O+ rises to 0,00001 (1 x 10-5) then the pH = 5. (Remember -7 is lower than -5.) From this, it can be observed that the concentration difference changes hundred-fold. This is, therefore, also the reason that even small differences can have significant implications when it comes to chemical reactions. A few typical pH values are for example, vinegar (pH = 3), coffee (pH = 5,5), baking soda (pH = 8) and bleach (pH = 13).
Certain active ingredients of products are sensitive to the hydroxyl ion’s concentration and others to the hydrogen ion’s concentration. This chemical reaction is known as hydrolysis. Hydrolysis is therefore a type of chemical reaction when water where the chemical bonds of the substrate dissolved or are broken. (From the Greek: hydro = water; lyse = break)
This changes the properties of the substrate in such a way that its original function can no longer be fulfilled. In pest-control terms, we can say that the active ingredient has been chemically broken down and will now show poor or ineffective control for the purpose it was applied.
How sensitive an active ingredient is towards pH is expressed in terms of its half-life. Each active ingredient will have its own timeline against which it becomes inactive. The time it takes for a concentrate’s active ingredient to become only half as effective is known as its half-life.
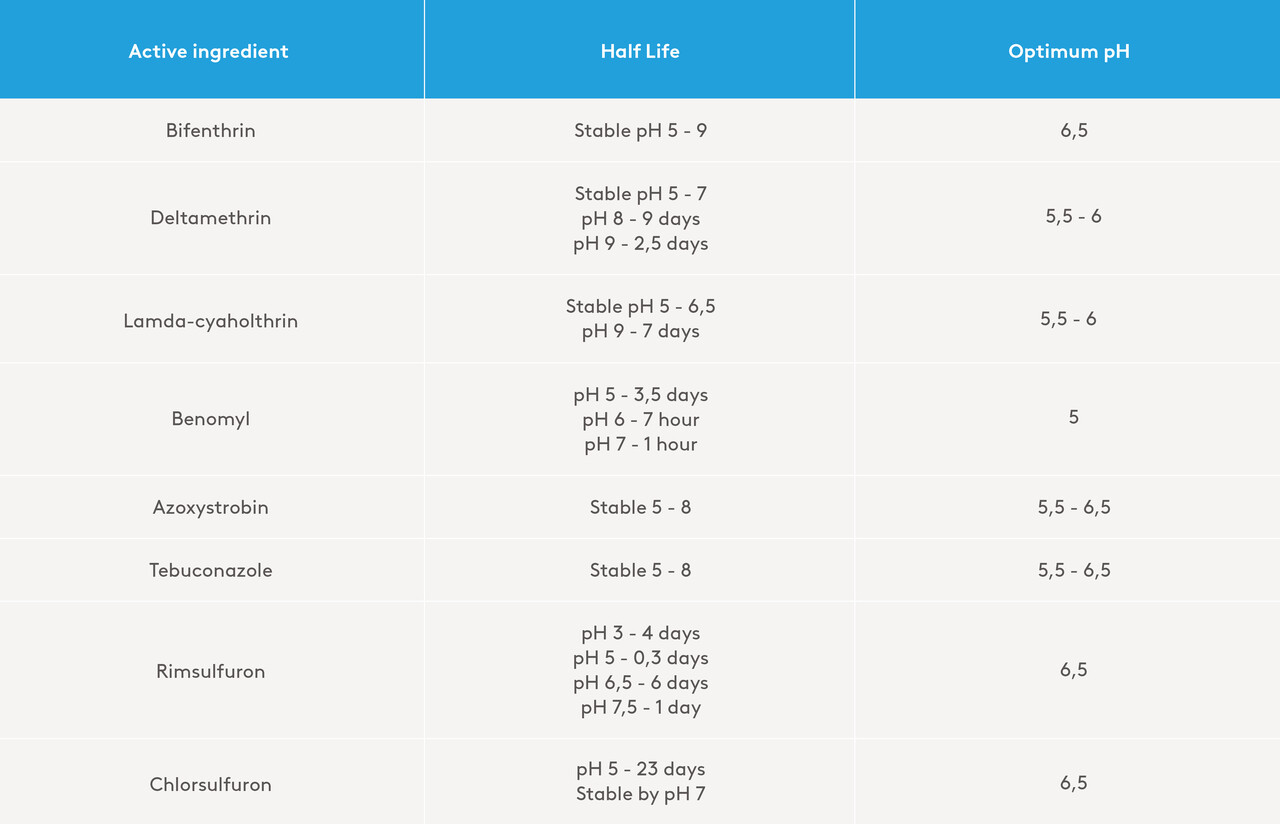
Table 2: Examples of half-lives at 20°C of a few active ingredients at specific pH levels. (2)
Fortunately, there are a wide variety of products available on the market to regulate water pH to the desired agricultural water quality standards. Titration water testing is suggested for water with high TDS or pH levels to determine accurate recommendations. Certain products are very effective at precipitating the salts and can leave nozzles clogged. In such cases, as a second best solution, only the pH of the spray mixture should be adjusted.
There are a few interesting characteristics to note from Table 2:
- Pyrethroids can vary in their pH requirement
- Some active ingredients break down at a low pH, while others break down at high pH values.
- Some half-lives are very short, while others are stable.
- Optimal pH ensures optimal effect. Occasionally, solubility can be affected by pH such as with saflufenacil (3).
From the information outlined above, it is clear that someone knowledgeable is required to make sense of all this information. Is it imperative that spray-water quality guidelines must be taken seriously. In part 2, water hardness and the effect of cations will be discussed.
Summary
- The information outlined above can be briefly summarised. The measuring of EC and pH is a critical part of determining the miscibility, efficacy and after-effects of the products used in a spray mixture.
- The water supply used for spraying must be tested as required and the necessary adjustments made for water conditioning.
- Farmers need to ensure that all products (including nutrients) added to a tank support the optimum pH for the best efficacy and after-effects of the active ingredients in the mixture.
- A buffer will not correct the pH in all instances, the use of the correct registered mixtures are essential to ensure that you as a farmer are getting value for your financial outlay and avoiding damage.
Resources:
1. Anna F. Rusydi, 2018, IOP Conf. Ser.: Earth Environ. Sci. 118 012019.
2. Table 2. and Figure 4. Various technical publications, SDS documents of active ingredients compiled for example, pH Stability Of Commonly Used Pesticides, by Allen D. Owings, Associate Specialist(Horticulture), Dale K. Pollet, Specialist (Entomology), Clayton A. Hollier, Specialist(Plant Pathology), Reed J. Lensce, Assistant Specialist (Weed Science), and Robert C. Trawick, Extension Associate (Horticulture), Louisiana State University, publications from New South Wales, HAL projects. https://grdc.com.au/, Grains and research, development corporation, Australia. Purdue University website.
3. Jared M. Roskamp, Ronald F. Turco, Marianne Bischoff, and William G. Johnson “The Influence of Carrier Water pH and Hardness on Saflufenacil Efficacy and Solubility,” Weed Technology 27(3), 527-533, (1 September 2013). https://doi.org/10.1614/WT-D-12-00154.1.